What You Should Know About CSV in Pharma
Por um escritor misterioso
Last updated 20 setembro 2024

Learn more about computer system validation, which is required by the FDA and other global regulatory bodies for drug and medical device manufacturers.
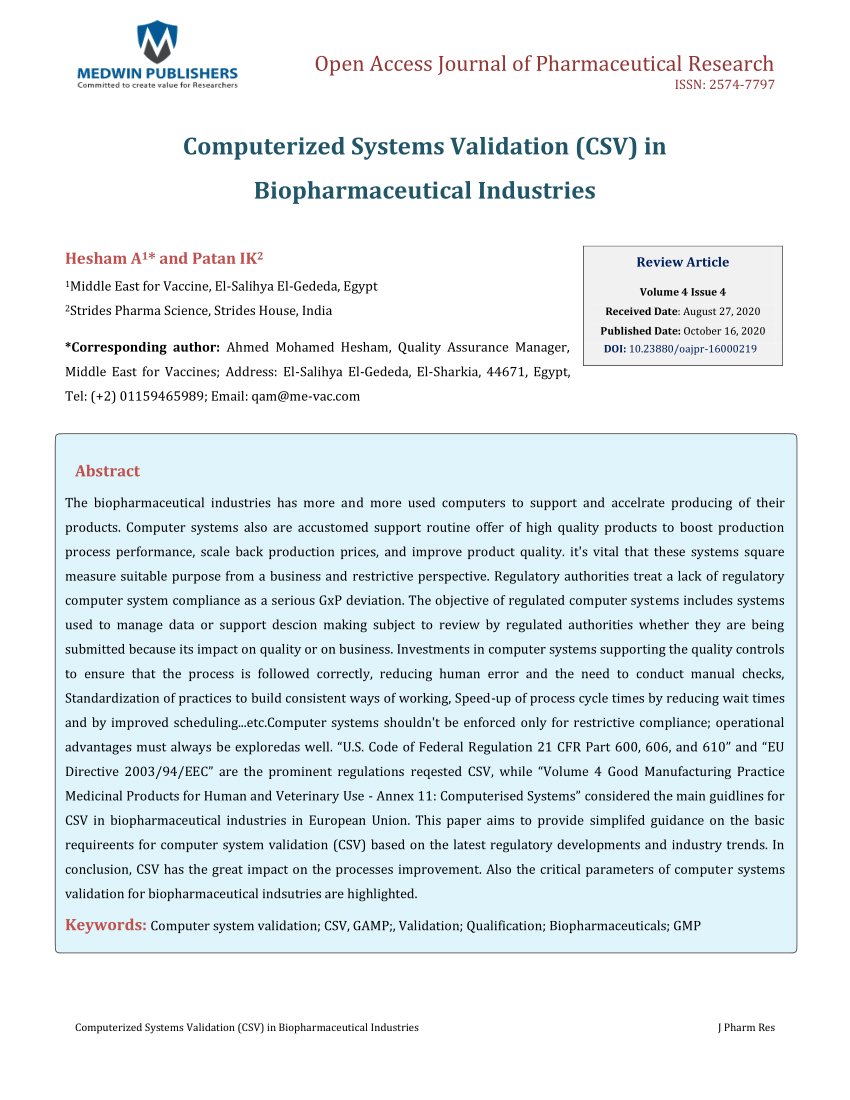
PDF) Computerized Systems Validation (CSV) in Biopharmaceutical Industries

Pharmaceutical Computer System Validation - CSV Validation in Pharma

Computer System Validation (CSV) in Life Sciences Part 1: Introduction to CSV - Verista

Computer System Validation in Pharmaceutical Industry

What You Should Know About CSV in Pharma

CSV vs. CSA: What Are the Main Differences?

Computer System Validation (CSV): Your Path to Documenting Data Integrity

A Guide to CSV & CSA - Why The Shift?
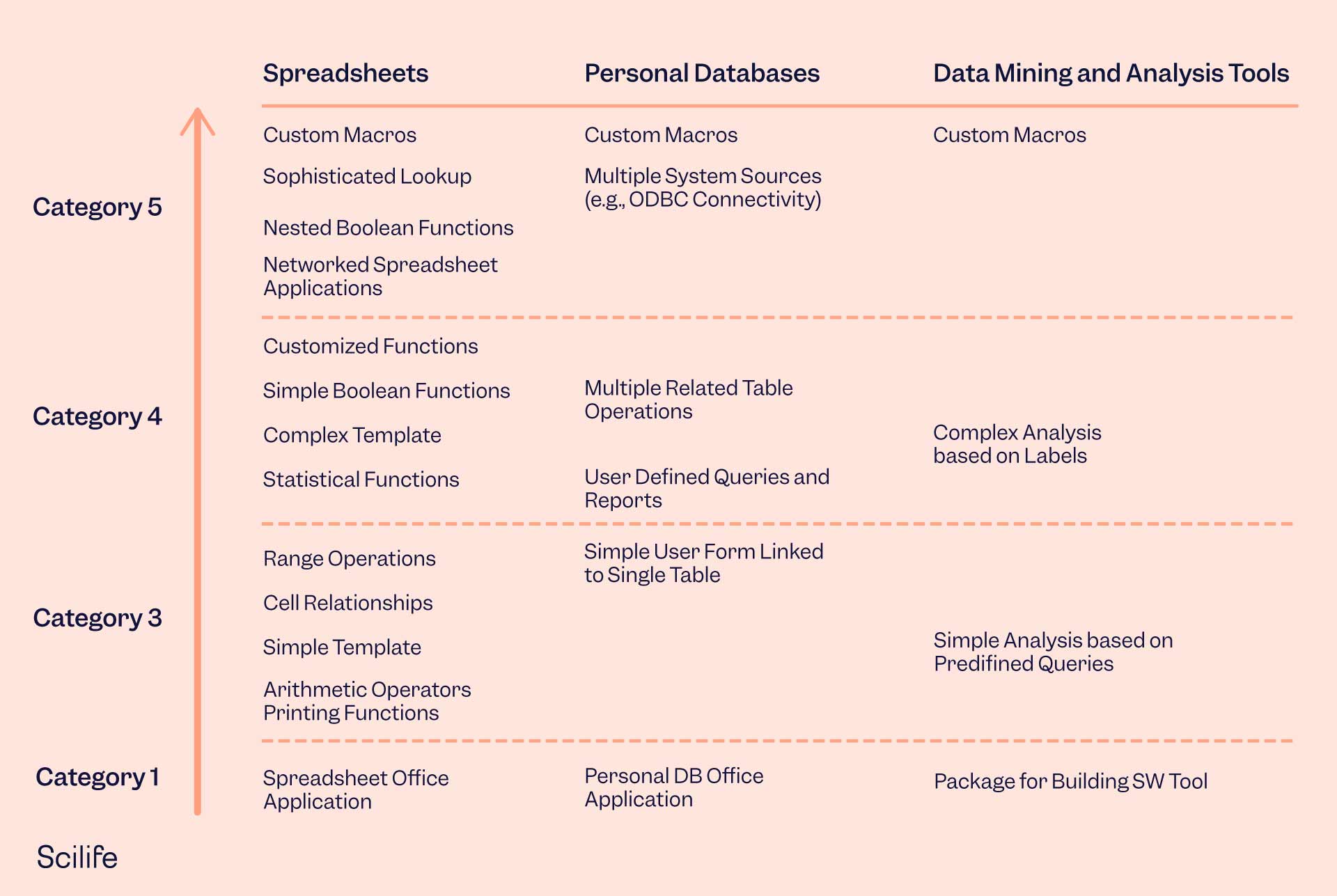
CSV vs. CSA: What Are the Main Differences?

Computer System Validation
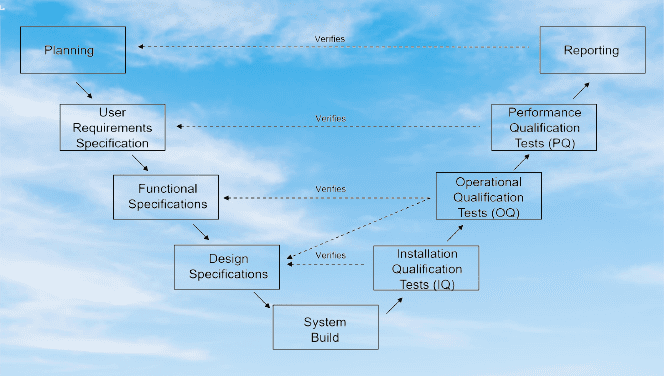
Computer System Validation (CSV) In Pharmaceuticals (23) » Flairpharma

Importance of computer system validation (CSV) in Pharmaceutical Industry
Strategic ChatGPT Prompts for Pharmaceutical Firm
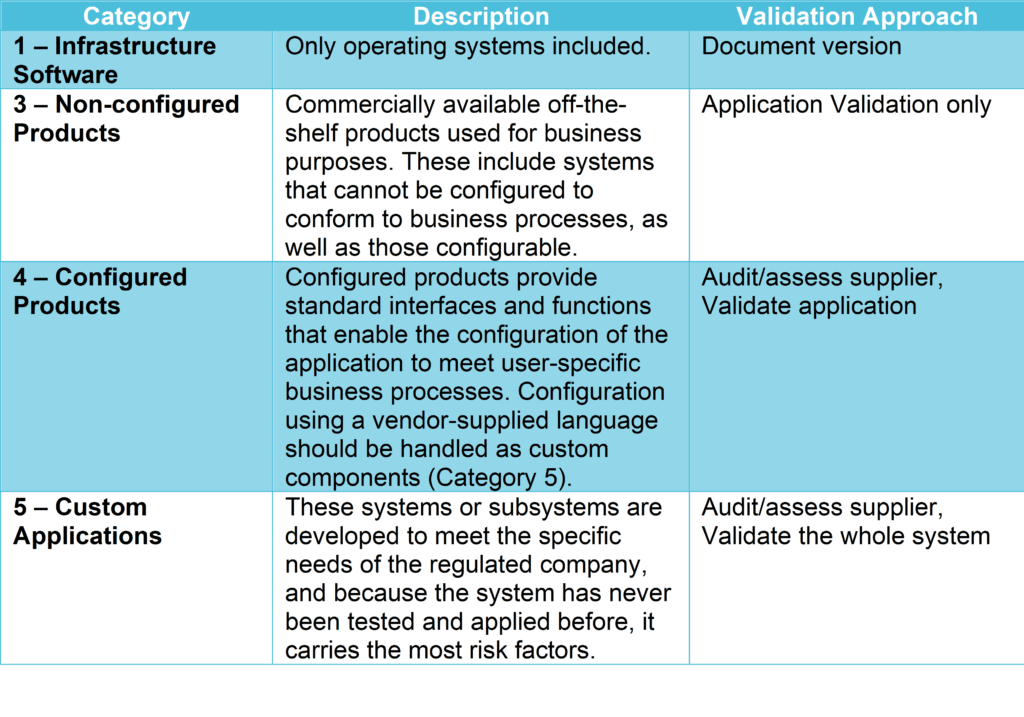
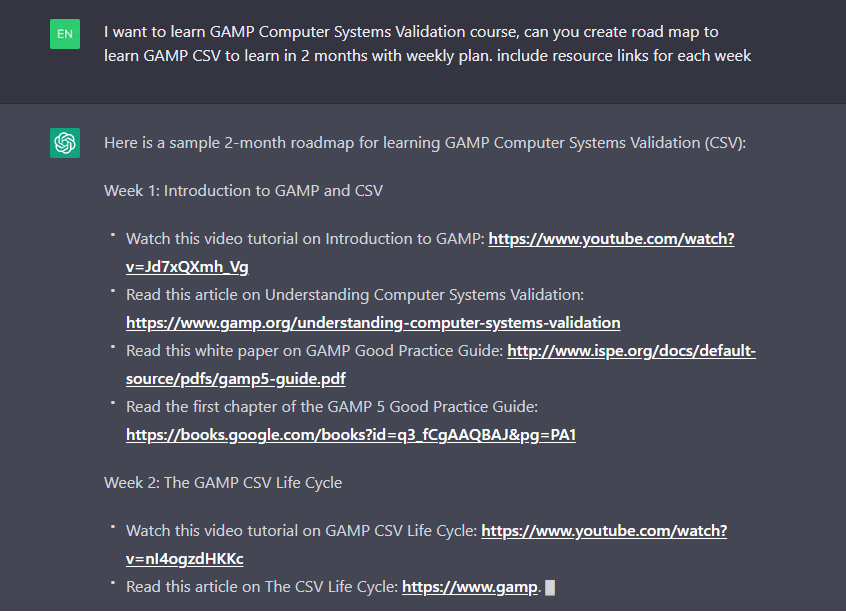
Risk-Based Computerized System Validation (CSV) and Computer Software Assurance (CSA) - Old Wine in a New Bottle? - Kvalito
Recomendado para você
-
 CRVPM Level V - Compliance Education Institute20 setembro 2024
CRVPM Level V - Compliance Education Institute20 setembro 2024 -
 CRVPM Level VI/Certified AICPA SOC® Report Analyst (CASRA20 setembro 2024
CRVPM Level VI/Certified AICPA SOC® Report Analyst (CASRA20 setembro 2024 -
 Addgene: pAAV-CAG-SomaGCaMP6f220 setembro 2024
Addgene: pAAV-CAG-SomaGCaMP6f220 setembro 2024 -
 CSV1A CYCLE STOP VALVE – Cycle Stop Valves, Inc20 setembro 2024
CSV1A CYCLE STOP VALVE – Cycle Stop Valves, Inc20 setembro 2024 -
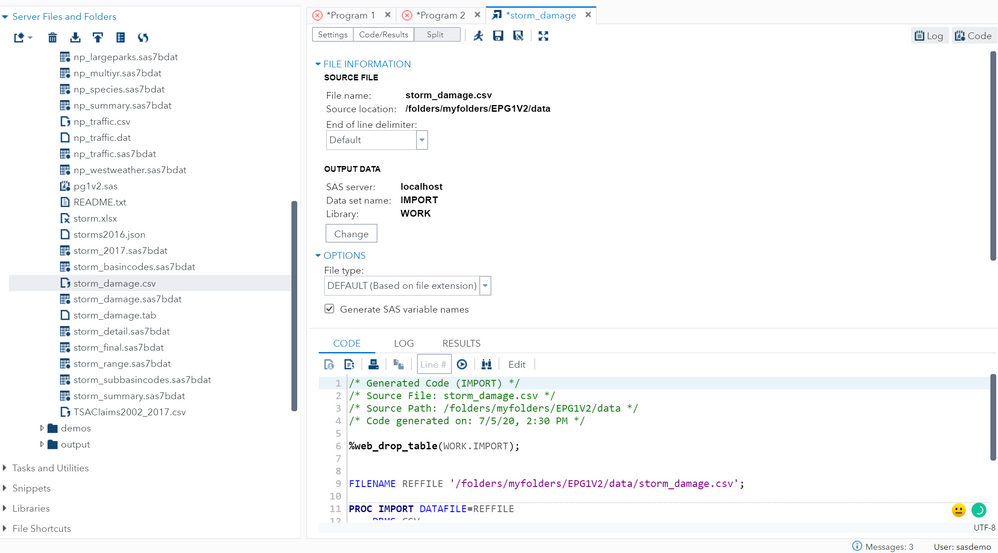
import csv file - SAS Support Communities20 setembro 2024
-
 Afiliados - SINEPE/MA20 setembro 2024
Afiliados - SINEPE/MA20 setembro 2024 -
 Feria Proland en el Eje Cafetero20 setembro 2024
Feria Proland en el Eje Cafetero20 setembro 2024 -
 Managing organizations via CSV Import — Zammad Admin Documentation20 setembro 2024
Managing organizations via CSV Import — Zammad Admin Documentation20 setembro 2024 -
 CSV File Format20 setembro 2024
CSV File Format20 setembro 2024 -
 What is CSV?20 setembro 2024
What is CSV?20 setembro 2024
você pode gostar
-
 Grave of the Fireflies - IGN20 setembro 2024
Grave of the Fireflies - IGN20 setembro 2024 -
 Why Is mail.facebook.com Pointing To An Outlook Web App Login?20 setembro 2024
Why Is mail.facebook.com Pointing To An Outlook Web App Login?20 setembro 2024 -
 Is Modern Warfare 2 (2009) worth playing in 2022?20 setembro 2024
Is Modern Warfare 2 (2009) worth playing in 2022?20 setembro 2024 -
 Gold Digger: कहां से आया ये गोल्ड डिगर20 setembro 2024
Gold Digger: कहां से आया ये गोल्ड डिगर20 setembro 2024 -
 Qué difícil es ver a una mujer que te incomoda”: Quién es el misterioso amor de Ana Isabel Araujo, la esposa de Pablo Lyle, que da señales de estar enamorada de nuevo20 setembro 2024
Qué difícil es ver a una mujer que te incomoda”: Quién es el misterioso amor de Ana Isabel Araujo, la esposa de Pablo Lyle, que da señales de estar enamorada de nuevo20 setembro 2024 -
Some of the best Online Slots and Top Casinos for Slot Games20 setembro 2024
-
 GIANTS IN A PREHISTORIC OCEAN!!! - Fish Feed and Grow20 setembro 2024
GIANTS IN A PREHISTORIC OCEAN!!! - Fish Feed and Grow20 setembro 2024 -
 Mortal Kombat PS Vita - Fatalities and Babalities list20 setembro 2024
Mortal Kombat PS Vita - Fatalities and Babalities list20 setembro 2024 -
Blue Lock's Deity (Blue Lock X Male Reader) - Episode 18 - The Stage for the Lead - Page 3 - Wattpad20 setembro 2024
-
 Monkey Mart - monkey manager android iOS apk download for free-TapTap20 setembro 2024
Monkey Mart - monkey manager android iOS apk download for free-TapTap20 setembro 2024