NDC Package 72266-103-01 Labetalol Hydrochloride Injection Intravenous
Por um escritor misterioso
Last updated 20 setembro 2024

Package of 1 vial, multi-dose in 1 carton / 40 ml in 1 vial, multi-dose of Labetalol Hydrochloride, a human prescription drug by Fosun Pharma Usa Inc..Labetal

Pfizer Hospital US

Labetalol Hydrochloride Injection, USP

Lidocaine HCl / Epinephrine 1% – 1:100,000 Injection MDV 50 mL (25

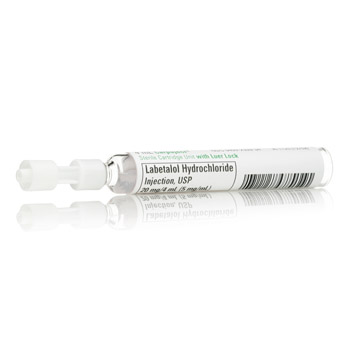
Labetalol Hydrochloride
Pfizer Hospital US
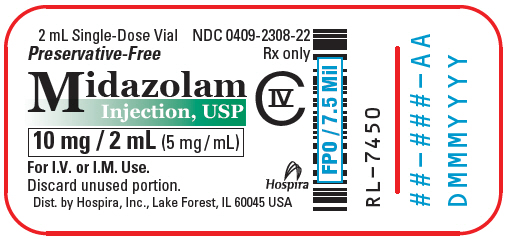
NDC Code 0409-2308-01 - Midazolam Hydrochloride

UDL Laboratories 51079093020 - McKesson Medical-Surgical

Labetal Injection

Almaject Inc 47781058656 - McKesson Medical-Surgical
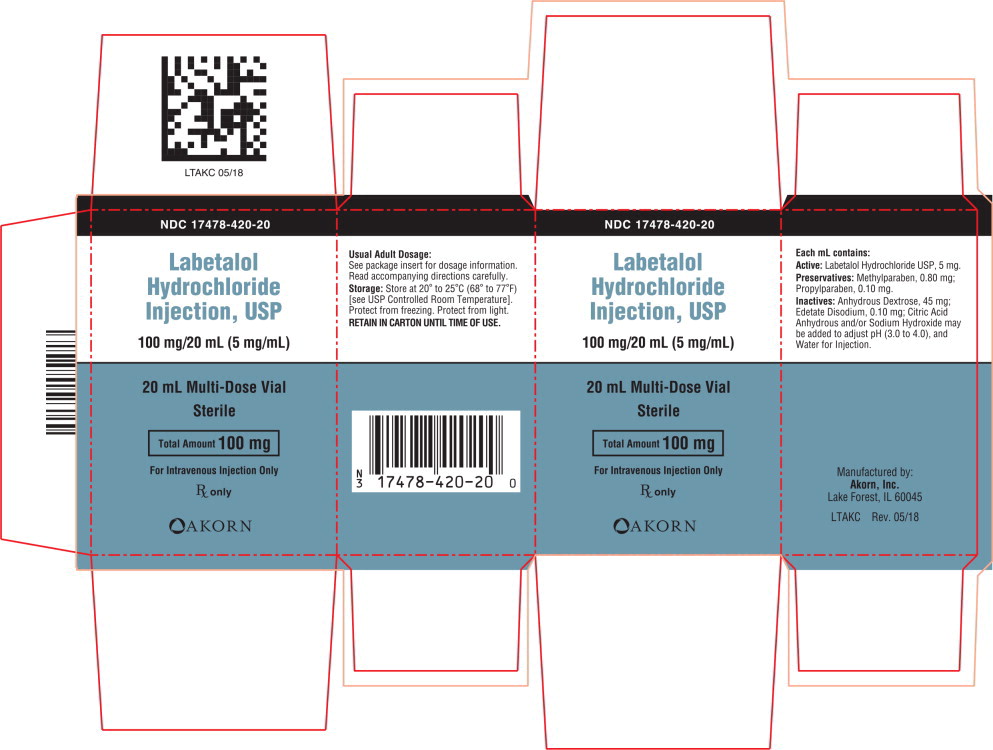
Labetalol Hydrochloride Injection, USP
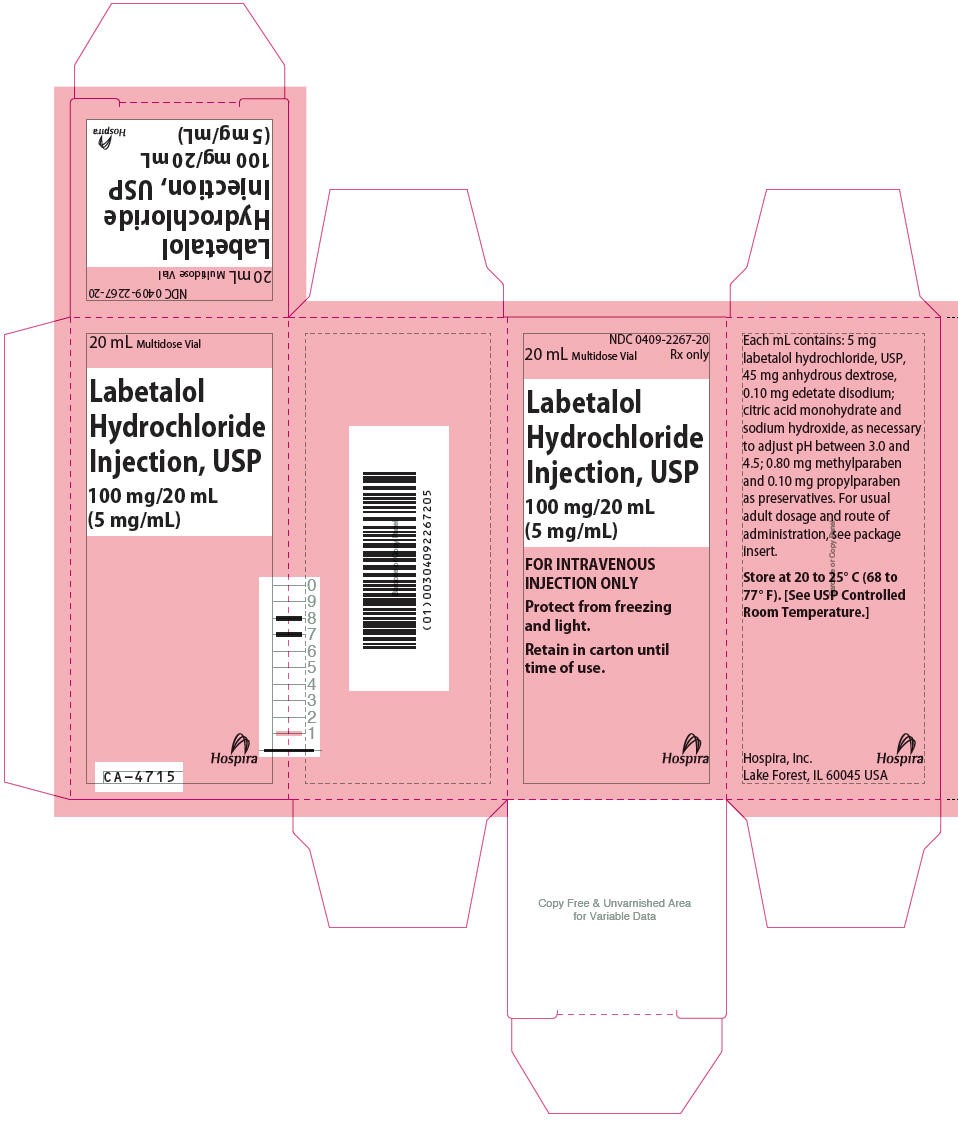
LABETALOL HYDROCHLORIDE INJECTION, USP 100mg/20mL (5mg/mL) VIAL
Fosun Products - Fosun Pharma USA
Recomendado para você
-
 Labetalol HCl Injection 5mg/mL MDV 20mL 20mL/Vl - Suprememed20 setembro 2024
Labetalol HCl Injection 5mg/mL MDV 20mL 20mL/Vl - Suprememed20 setembro 2024 -
 Labet Injection – Generix Global Investment Ltd20 setembro 2024
Labet Injection – Generix Global Investment Ltd20 setembro 2024 -
 Labetalol: Side Effects, Dosage, Uses, and More20 setembro 2024
Labetalol: Side Effects, Dosage, Uses, and More20 setembro 2024 -
 LABESOL Labetalol HCl Injection USP » SGPharma20 setembro 2024
LABESOL Labetalol HCl Injection USP » SGPharma20 setembro 2024 -
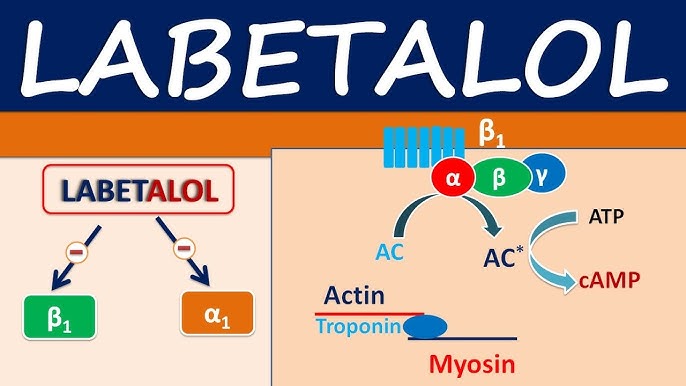 Labetalol 100 mg (Trandate): What Is Labetalol Used For? Uses, Dosage and Side Effects of Labetalol20 setembro 2024
Labetalol 100 mg (Trandate): What Is Labetalol Used For? Uses, Dosage and Side Effects of Labetalol20 setembro 2024 -
 LABETALOL HYDROCHLORIDE INJECTION, USP20 setembro 2024
LABETALOL HYDROCHLORIDE INJECTION, USP20 setembro 2024 -
 Labetalol Hydrochloride Injection, USP, 20 MG/ 4ML20 setembro 2024
Labetalol Hydrochloride Injection, USP, 20 MG/ 4ML20 setembro 2024 -
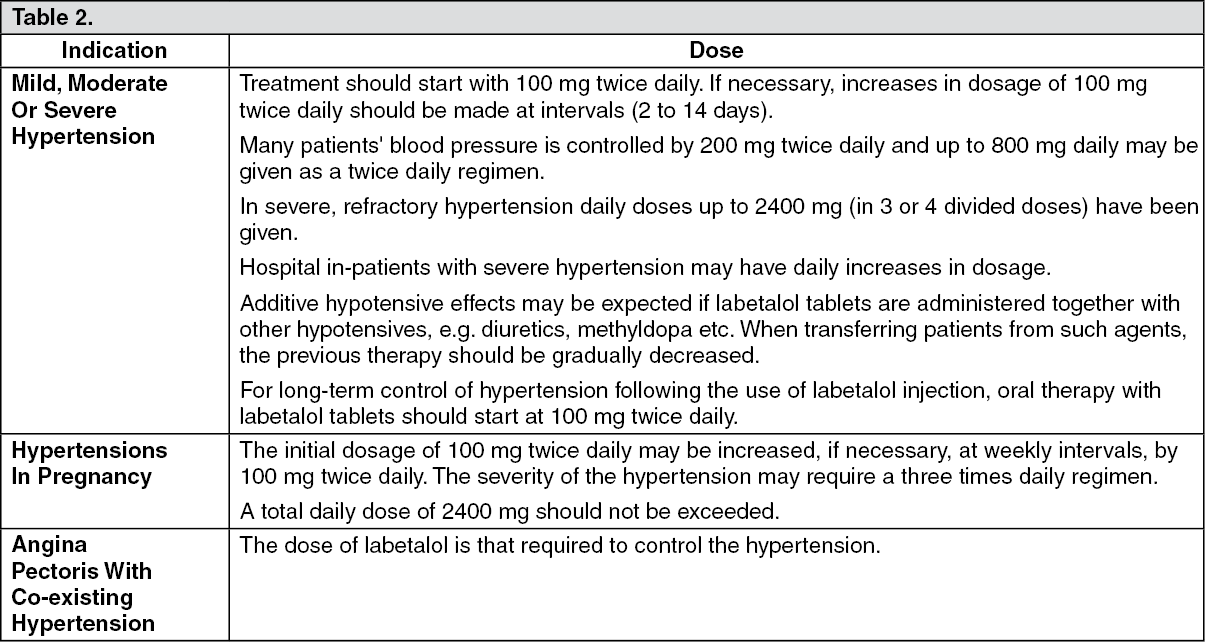 Trandate Full Prescribing Information, Dosage & Side Effects20 setembro 2024
Trandate Full Prescribing Information, Dosage & Side Effects20 setembro 2024 -
 LABETALOL: Uses, Side Effects and Medicines20 setembro 2024
LABETALOL: Uses, Side Effects and Medicines20 setembro 2024 -
 LABETALOL HCL 200MG MDV 40ML - HIKMA PHARMACEUTICALS USA INC20 setembro 2024
LABETALOL HCL 200MG MDV 40ML - HIKMA PHARMACEUTICALS USA INC20 setembro 2024
você pode gostar
-
 7 Pool Table Games That Are Currently Popular20 setembro 2024
7 Pool Table Games That Are Currently Popular20 setembro 2024 -
 Emojiology: 😏 Smirking Face20 setembro 2024
Emojiology: 😏 Smirking Face20 setembro 2024 -
 Top 5 meta weapons in Warzone 2's Ashika Island in Season 220 setembro 2024
Top 5 meta weapons in Warzone 2's Ashika Island in Season 220 setembro 2024 -
 League of Legends: How Xbox Game Pass Does (and Doesn't) Save You20 setembro 2024
League of Legends: How Xbox Game Pass Does (and Doesn't) Save You20 setembro 2024 -
 Mulher-Hulk Série teve orçamento surpreendente20 setembro 2024
Mulher-Hulk Série teve orçamento surpreendente20 setembro 2024 -
 Anitube20 setembro 2024
Anitube20 setembro 2024 -
 Desenhos Animados Ilustração Jogo Educativo Peças Quebra Cabeça20 setembro 2024
Desenhos Animados Ilustração Jogo Educativo Peças Quebra Cabeça20 setembro 2024 -
 Piano Teclado Infantil Microfone Karaoke Brinquedo Musical - Fun th - Piano / Teclado de Brinquedo - Magazine Luiza20 setembro 2024
Piano Teclado Infantil Microfone Karaoke Brinquedo Musical - Fun th - Piano / Teclado de Brinquedo - Magazine Luiza20 setembro 2024 -
 yagate kimi ni naru koito yuu seifuku tagme, #106158020 setembro 2024
yagate kimi ni naru koito yuu seifuku tagme, #106158020 setembro 2024 -
 A história de Harry Potter nos games - Canaltech20 setembro 2024
A história de Harry Potter nos games - Canaltech20 setembro 2024